24-January-2024
Chemical vapor deposition (CVD) is a process that involves depositing a solid material from a gaseous phase (often diluted in carrier gases), which differs from the PVD process that the precursors are solid, with the material to be deposited being vaporized from a solid target and deposited onto the substrate. It roughly consists of three steps:
(1) Formation of volatile substances;
(2) Transfer the above materials to the deposition area;
(3) Produce chemical reactions on solids and produce solid substances.
The most basic chemical vapor deposition reactions include thermal decomposition reactions, chemical synthesis reactions, and chemical transport reactions.
Among them, pyrolytic graphite crucible and pyrolytic boron nitride crucible are both prepared through the thermal decomposition reaction of this technology. Therefore, these two crucibles have some common performance characteristics, such as high temperature resistance, wear resistance, and high purity. Obviously, the biggest difference between them is that they use different materials, so their product properties are also different, so that the fields of use of the two crucibles are also different.

CVD-1200 system
What are the specific differences between these two crucibles that use the same preparation method? Below we will learn about the differences between pyrolytic graphite crucibles and pyrolytic boron nitride crucibles in terms of their preparation processes, characteristics and applications.
1. Pyrolytic graphite (PG) crucible
Pyrolytic graphite crucible is prepared from a new type of carbon material - pyrolytic graphite. Pyrolytic graphite is a high-purity hydrocarbon gas that is chemically vaporized on a graphite matrix of 1800°C to 2000°C under a certain furnace pressure. Deposited pyrolytic carbon of higher crystallographic orientation. It has high density (2.20g/cm³), high purity (impurity content (0.0002%)) and anisotropy of thermal, electrical, magnetic and mechanical properties. It can still maintain a vacuum of 10mmHg at around 1800°C. Therefore, this material The prepared crucible has better performance, such as better thermal conductivity, stronger corrosion resistance to acidic and alkaline solutions, smoother and denser surface, lower permeability, etc., and has wider application prospects.

01 Preparation process
Pyrolytic graphite crucible is different from ordinary graphite crucible. It is made by cracking hydrocarbons under high temperature, low pressure, and nitrogen atmosphere, so that carbon atoms are directionally deposited on the model, and then demoulded after cooling.
02 Main features
(1) High temperature resistance and low ablation rate;
(2) Resistant to acid and alkali chemical corrosion at high temperatures (700°C);
(3) The surface is smooth, dense, has low permeability, and is easy to clean and decontaminate;
(4) Have certain mechanical strength;
(5) High purity, no contamination of samples, and can be used repeatedly.
03 Application
Pyrolytic graphite crucible can not only be used for the pretreatment of samples in minerals, building materials, glass, etc., but can also be used in the preparation of high-purity metal oxides, ion plating, etc. For example, some researchers used pyrolytic graphite (PG) crucibles instead of platinum, quartz and other crucibles to prepare high-purity yellow lead oxide and achieved ideal results.
2. Pyrolytic boron nitride (PBN) crucible
Boron nitride crucibles are usually composed of PBN. PBN ceramics have good heat resistance, thermal stability, thermal conductivity, and high-temperature dielectric strength, and are ideal heat dissipation materials and high-temperature insulation materials.

Boron nitride powder
Due to PBN's excellent chemical stability, it can resist the erosion of most molten metals, and because of the above-mentioned high-temperature insulation properties, high thermal conductivity, and low thermal expansion, it is most suitable for use under strict environmental conditions such as semiconductor manufacturing processes. Materials used. PBN crucible is often used for smelting metals and semiconductors. Its operating temperature can reach up to 1800 degrees under vacuum and up to 2100 degrees under atmosphere protection. It is generally protected by nitrogen or argon (atmosphere protection is to prevent the crucible from oxidizing).
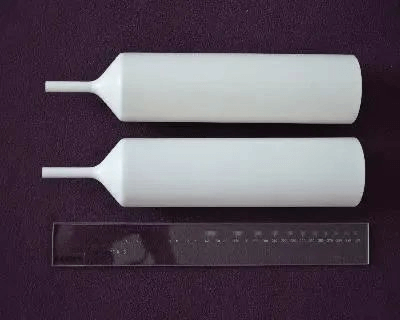
PBN Crucibles
01 Preparation process
(1) The boron-containing gas compound and nitrogen-containing gas are subjected to a chemical vapor deposition reaction on a heated mold core under vacuum conditions to obtain a pre-coating;
(2) After obtaining the pre-coating, stop the chemical vapor deposition reaction and maintain a constant temperature;
(3) After maintaining constant temperature, continue the chemical vapor deposition reaction to obtain a pyrolytic boron nitride crucible with pre-coating;
(4) Remove the pre-coating to obtain a pyrolytic boron nitride crucible.
02 Main features
(1) Excellent chemical and thermal stability. Does not react chemically with most molten metals and semiconductors at high temperatures;
(2) Pyrolytic boron nitride crucible has high density and no pores;
(3) Can be used repeatedly and is easy to clean;
(4) It has obvious anisotropy in terms of mechanical, thermal and electrical properties, and also has good properties of transmitting microwaves and infrared rays;
(5) Good high temperature insulation performance.
03 Application
Since the PBN crucible does not need to go through the traditional hot-pressing sintering process and does not need to add any sintering agent, it has a very high purity (more than 99.99%). The use temperature under vacuum can be as high as 1800 degrees, and the use temperature under atmosphere protection can be as high as 2100 degrees Celsius. , (usually nitrogen or argon), mostly used in evaporation/molecular beam epitaxy (MBE)/GaAs crystal growth and other purposes. In addition, due to the slow deposition speed, PBN crucibles are quite expensive and are mostly small-sized crucibles.
Pyrolytic graphite crucible and pyrolytic boron nitride crucible use the same preparation technology, so they have some similar performance characteristics. However, due to different materials, they each have unique properties and applications.
Pyrolytic graphite crucible has the characteristics of low ablation rate, resistance to acid and alkali chemical corrosion at high temperatures (700°C), density, low permeability, and easy cleaning and decontamination. It can be used as mineral raw materials, building materials, high-purity materials, Analysis vessels for grain and feed samples can also partially replace crucible products made of platinum, silver, nickel, corundum, polytetrafluoroethylene, graphite and other materials.
Pyrolytic boron nitride crucible has the characteristics of high chemical purity, good thermal conductivity and insulation, obvious anisotropy, and can be processed, and can be used for evaporation/molecular beam epitaxy/liquid phase epitaxy/arsenic Gallium single crystal preparation and other uses also play an irreplaceable role in many cutting-edge material preparation fields. For example, the pyrolytic boron nitride crucible has more advantages in the growth of CdZnTe crystals. The pyrolytic boron nitride crucible has excellent performance, does not react with CdZnTe, and has a large wetting angle. These properties are more conducive to the preparation of high-quality CdZnTe single crystals. , this crystal is widely used as the epitaxial substrate of infrared detector HgCdTe and room temperature nuclear radiation detectors.